PPAR agonists
Peroxisome proliferator-activated receptors (PPARs) are a group of nuclear receptor proteins that act as transcription factors for gene expression by binding to specific DNA sequences. They have been shown to have a role in several physiological processes including carbohydrate and lipid metabolism as well as inflammation and fibrogenesis, and represent a promising target for therapy.
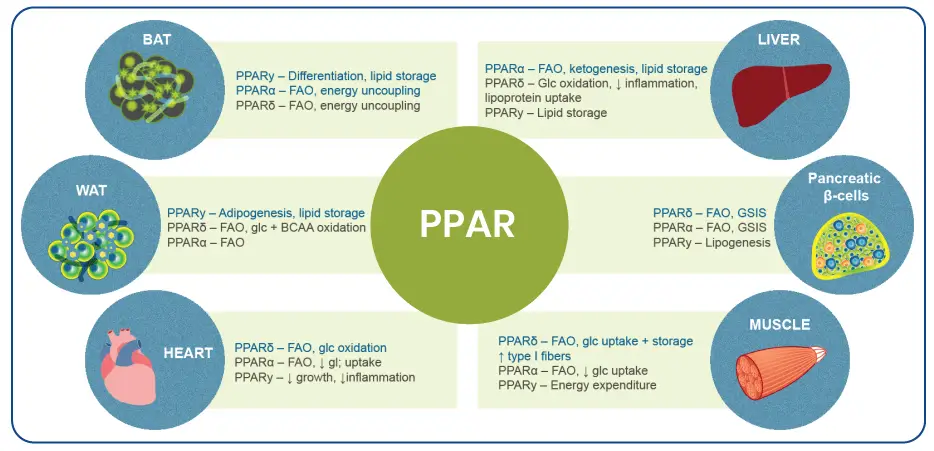
The PPAR family comprises three isoforms: PPARα, PPARβ/δ and PPARγ, which are differentially expressed in various tissues.
PPARα is expressed ubiquitously but most highly in the liver. PPAR-α plays a critical role in the regulation of fatty acid transport and β-oxidation and triglyceride turnover, is involved in regulation of energy homeostasis and is thought to have anti-inflammatory effects through complex regulation of nuclear factor kappa-light-chain-enhancer of activated B-cells (NF-κB) and activator protein 1 (AP1) transcription factors.
PPARβ/δ is expressed mainly in skeletal muscle and to a lesser degree in adipose tissue and liver. PPARβ/δ contributes to the regulation of glucose and lipid metabolism and is involved in regulating mitochondrial metabolism and fatty acid β-oxidation in muscle. It is also present in Kupffer cells and HSCs, and may, therefore, have a role in inflammation and fibrosis since HSCs as well as portal fibroblasts are the main source of hepatic myofibroblasts. Furthermore, PPARβ/δ is also expressed in vascular cells including endothelial cells, smooth muscle cells and macrophages, and it plays an important role in various basic vascular processes such as apoptosis, survival, angiogenesis and inflammation.
PPARγ is highly expressed in adipose tissue and plays an essential role in the regulation of adipocyte differentiation, adipogenesis and lipid metabolism; its activation induces insulin sensitization, enhances glucose metabolism, increases triglyceride storage in adipocytes while decreasing liver steatosis in humans. In addition, it stimulates secretion of the anti-inflammatory cytokine adiponectin. PPARγ is also expressed in HSCs where it plays a key role in maintaining them inactive. Furthermore, forced expression of PPARγ has been shown to reverse activated HSCs to their quiescent state.
Both PPARα-β/δ dual agonism as well as PPARγ agonism have shown beneficial effects on liver histology in phase IIb clinical trials for NASH. Single, dual and pan-PPAR agonists are under development for the pharmacological treatment of NASH.
RELATED PPARs VIDEOS

NASH 2024: The New Nomenclature and Revised Definition
Dive into the transformative world of liver health with Prof. Vlad Ratziu’s enlightening insights on

Clinical Chemistry In NAFLD Part 3 – Biomarkers and Hepatic Fibrosis
Discover the intricate dynamics of Clinical Chemistry in NAFLD with Dr. Guillaume Grzych, a leading

Clinical Chemistry in NAFLD Part 2 – Biomarkers, Hepatic Steatosis and NASH
Discover how key biomarkers like Fatty Liver Index and SteatoTest are transforming NAFLD diagnosis and

Clinical Chemistry in NAFLD Part 1 – Interest and Why?
Explore the complexities of NAFLD (Non-Alcoholic Fatty Liver Disease), now also known as MAFLD, and
RELATED PPARs ARTICLES
Hepatic inflammatory responses in liver fibrosis
Recent technological advancements have provided unprecedented insights into the inflammatory mechanisms underlying liver fibrosis, leading
Screening, Diagnosis, and Staging of Non-Alcoholic Fatty Liver Disease (NAFLD): Application of Society Guidelines to Clinical Practice
Metabolic dysfunction-associated steatotic liver disease (MASLD), formerly known as non-alcoholic fatty liver disease (NAFLD) has
Surveying Stigma: MAFLD’s Nomenclature from Patient and Provider Perspectives
Non-alcoholic fatty liver disease (NAFLD) is estimated to affect 38% of adults worldwide, a statistic
Cardiovascular Disease in MASLD Patients: Mitigating Risks
The term metabolic dysfunction-associated steatotic liver disease (MASLD) has recently been proposed as an alternative
References
- Derosa G, Sahebkar A, Maffioli P. The role of various peroxisome proliferator-activated receptors and their ligands in clinical practice. Journal of cellular physiology. 2018;233(1):153-61.
- Francque S, Szabo G, Abdelmalek MF, et al. Nonalcoholic steatohepatitis: the role of peroxisome proliferator-activated receptors. Nat Rev Gastroenterol Hepatol. 2021 Jan;18(1):24-39.
- Hong F, Xu P, Zhai Y. The opportunities and challenges of peroxisome proliferator-activated receptors ligands in clinical drug discovery and development. Int J Mol Sci. 2018;19(8).
- Liss KH, Finck BN. PPARs and nonalcoholic fatty liver disease. 2017;136:65-74
- Tyagi S, Gupta P, Saini AS, Kaushal C, Sharma S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J Adv Pharm Technol Res. 2011;2(4):236-40.
- Wettstein G, Luccarini JM, Poekes L, Faye P, Kupkowski F, Adarbes V, et al. The new-generation pan-peroxisome proliferator-activated receptor agonist IVA337 protects the liver from metabolic disorders and fibrosis. Hepatol 2017;1(6):524-37.