OVERVIEW OF Non-Invasive Assessment of cACLD and Portal Hypertension
Table of Contents
- What’s new in the assessment of cirrhosis?
- The paradigm of portal hypertension has shifted over the last years
- Factors Associated With Liver-Related Clinical Events in Patients With Cirrhosis
- From histology to the non-invasive definition of advanced liver disease
- The progressiveness of liver disease severity according to liver stiffness
- Baveno criteria
- My case
- Case presentation in 2022
- Non-invasive tests
- My case – Transient elastography
- Baveno VII
- Changes of liver stiffness over time in advanced liver disease
- Prognostic evidence of benefit from NSBB therapy
- Adding spleen stiffness to stratify patients’ cACLD
- Case presentation Spleen Stiffness by TE (100 Hz exam)
- Direct Fibrogenesis markers available in clinic
- Cirrhosis or no cirrhosis?
- Case presentation – History
- Laparoscopy – Taking a peek at the river
- Non-Invasive Assessment of cACLD and Portal Hypertension
Hello everyone. My name is Prof. Dr. Jörn M. Schattenberg. I’m the director of the metabolic liver research program at the University Medical Center in Mainz. It’s my pleasure to give this short presentation on non-invasive assessment of compensated advanced chronic liver disease and portal hypertension within the PanNASH initiative. I hope you enjoy the presentation. If you have any questions or feedback, feel free to reach out. Many aspects have been happening in the field, and I want to introduce some of the more recent concepts to you.
What’s new in the assessment of cirrhosis?
All of you probably know liver cirrhosis as a disease very well and have a concept of how to manage and diagnose the disease. However, there are certain barriers and thresholds in the field, in particular, if you rely on liver histology to assess liver sclerosis as such, I think it’s timely to advance the field by choosing non-invasive biomarkers to assess liver cirrhosis, and I’m going to be talking to you on this.
The paradigm of portal hypertension has shifted over the last years
So, over the last years, we’ve seen a paradigm shift in portal hypertension. Portal hypertension per se is an HVPG-driven concept, meaning the pressure that is existent in the portal venous tract drives outcome in patients with one to five-millimeter mercury pressure being normal blood pressure and a normal right heart pressure and six to nine-millimeter mercury or preclinical sinusoidal portal hypertension. Whereas, starting at 10 millimeter mercury, we consider clinically significant portal hypertension to be present.
This is relevant because starting at 10 millimeter mercury, CSPH is present and is associated with oesophagal varices, clinical decompensating events, or even hepatocellular carcinoma in such outcomes. Therefore, stratifying patients according to their HVPG is logical or clinically relevant.
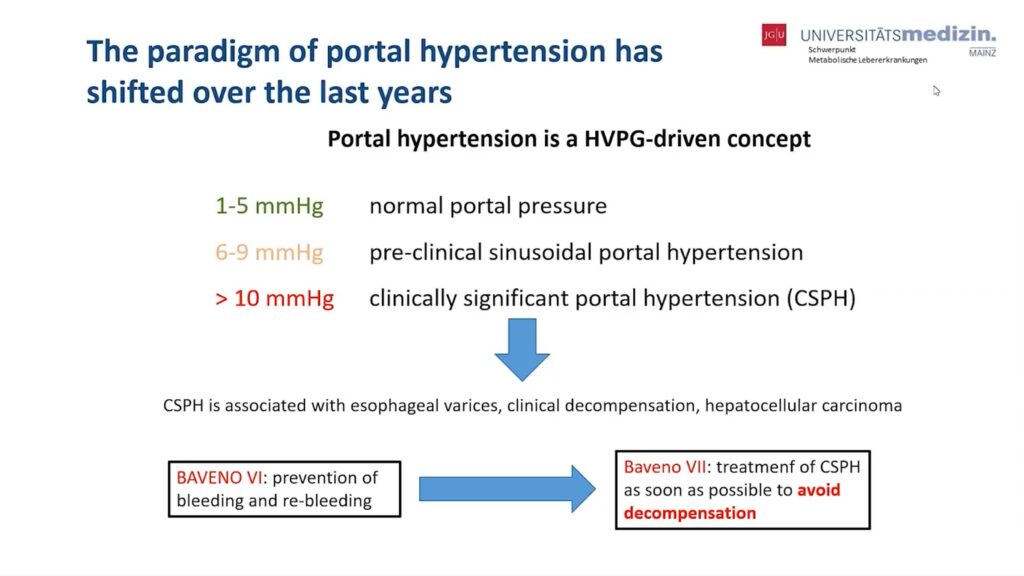
There’s been a number of consensus conferences, so-called Baveno conferences, around this issue, and we’ve seen a shift in their focus: while Baveno VI was mainly focused on the prevention of bleeding and complications of portal hypertension, now Baveno VII criteria, which have been released recently in the Journal of Hepatology, focus more on the treatment of compensated significant portal hypertension to avoid decompensation.
I’m going to show you some of the concepts that emerged from those Baveno conferences in the following slides.
Factors Associated With Liver-Related Clinical Events in Patients With Cirrhosis
First of all, as I said, I think it’s valid to stratify patients according to HVPG or portal pressure. In this clinical trial, as you can see here, it’s in the summary of the Simtuzumab studies. Simtuzumab was trialled for the indication of NASH, pre-cirrhotic NASH and cirrhotic NASH, and was a negative trial in the end. However, the authors analysed and stratified patients according to baseline HVPG and followed them, assessing event-free survival. And that’s shown in the right-hand graph here over the time frame of over 48 months.
And you can see that liver-related clinical events in the patients with cirrhosis occurred according to baseline HVPG and an increase of 1 millimeter mercury in HVPG was associated with an increased event rate, and as such, that cutoff that defines clinically significant portal hypertension – ten millimeter mercury – is relevant to our patients because of the clinical outcomes it predicts.
A number of different non-invasive tests can be used to replicate that. And one of the advantages that has been made in the field is again to move away from histology and use these non-invasive biomarkers for what’s currently considered compensated advanced chronic liver disease, subdividing pre-cirrhotic and cirrhotic conditions based on NIT cutoffs.
On the right-hand side, you can see this is taken from a study. The number of biomarkers were compared to histology – transient elastography and enhanced liver fibrosis (ELF) – and you can see that the survival curve, the liver-related event, or time free of liver-related events, pairs up nicely in the groups – histological stage F0 and F1, below 10 kilopascals and an ELF score below 9.8.
Whereas in the intermediate categories, transient elastography ranged from 10 to 15 kilopascals and ELF 9.8 till 10.5. Or in the pre-cirrhotic and cirrhotic stages, transit elastography above 15 kilopascals, ELF above 10.5. Those are the groups with the worst survival. So it’s well-suited to actually use non-invasive tests to stratify patients based on their outcome or their likelihood of events.
So on this slide, you can see that beyond HVPG, there is the opportunity to actually risk stratify patients based on non-invasive markers, according to compensated advanced chronic liver disease. On the right-hand side is a study that actually groups patients based on histology, transient elastography and ELF. There are three groups, and you can see that they share the event rate over the time frame of 7.5 years and it, well, groups together for the patients that are cirrhotic in histology and have transient elastography above 15 or an ELF above 10.5.
And as such, based on what I said, it’s valid to actually leave liver histology, HVPG, invasive assessments using non-invasive biomarkers to risk stratify these patients. I’d like to expand that concept a little further, looking at transient elastography and liver stiffness measurement to risk stratify patients. Between 5 and 10 kilopascals, you can exclude compensated advanced chronic liver disease, whereas above 20 kilopascals you can assume compensated advanced chronic liver disease.
And even above 25 kilopascals, you can rule in clinically significant portal hypertension. And this is considered the rule of five: sub-segmenting transient elastography readings into those categories of five with incremental worsening of prognosis and severity of liver disease, even irrespective of etiology.
And therefore, again, we are in the position today to use non-invasive tests to risk stratify patients with compensated advanced chronic liver disease. This has been published in the Baveno criteria and again shows the entire graph of what I’ve shown you before here. If you have a liver stiffness below 10, you can rule out compensated advanced chronic liver disease.
Whereas a liver stiffness of about 15, this is highly suggestive of compensated advanced chronic liver disease. Clinically significant portal hypertension, the end of the spectrum of liver cirrhosis, can be ruled in above 25 kilopascals and ruled out if transient elastography is below 15 and platelets are above 150,000. So let So let me bring this into my clinic, and for that, I’ve brought along a case that I’d like to show and discuss with you.
This is a 70-year-old woman that presented to me in 2022. She has diabetes mellitus that is well treated. She has arterial hypertension. She’s overweight, but not obese. And six years back, I did do a liver biopsy on her, seeing steatosis grade 3, activity grade 3, with 1 in inflammation and 2 in ballooning and intermediate fibrosis stage F2.
She does consume alcohol, but at a level that you would consider her as a patient suffering from non-alcoholic fatty liver disease. And her labs are shown on the right-hand side. You can see these slightly elevated transaminases, GGT is 37, glucose is not normal, A1C is 7.4 and no thrombocytopenia.
So the question is, then, are we worried about cirrhosis in this patient?
So how can I best assess the presence of compensated advanced chronic liver disease in this patient? Something that I tell my referring physicians to decide on this is to use the FIB-4 score. The FIB-4 score is pretty much available in most clinical settings where lab values are present. It combines age, AST, platelets and ALT in a formula that gives you a low cutoff of advanced liver disease and this has a good specificity. And it gives you a high cutoff with a high probability of F3 and F4 disease. However, here, the positive predicted value is clearly not as good as the negative predictive value at the lower cutoff.
And my patient has 1.75, which puts her into an intermediate range, and we cannot more specifically rule her in or rule her out. And as such, follow-up NITs assessment is required in this patient if I see her again after that time.
And the test we’re frequently doing and that’s been suggested by the Baveno criteria II is transient elastography. You can see here, a reading of 11.1 kilopascals, a median of more than 10 measurements and a top value of 308 showing us that she has fatty liver disease with at least suspected compensated advanced chronic liver disease.
Does that rule in cirrhosis? Please remember the rule of five that is suggested. No, it does not. The Youden index that was calculated and that rules in cirrhosis in a British cohort was 13.6. So clearly this patient is also below this and most likely she did not have cirrhosis according to this NIT.
And this is again how you can picture her into currently suggested algorithm. She is in the range between 10 and 15 kilopascals, which is suggestive of advanced compensated, advanced chronic liver disease, but we can rule out clinical significant portal hypertension and probably even rule out cirrhosis because she’s below 15 kilopascals.
Anyway, I think that the differentiation of these patients that are between 10 and 15 kilopascals is not always easy and therefore potentially additional testing is warranted if we need to establish the presence or absence of liver cirrhosis.
It’s also important to remember that the changes in liver stiffness over time, and this is a study that’s been published in Clinical Gastroenterology Hepatology in 2021, is of relevance. So, I saw that patient 6 years apart with FibroScan at baseline and over time I can again risk stratify patients.
You can see here the cumulative rate of liver decompensation shown according to a change of liver stiffness of less than 20% or in the yellow line, more than 20% and these patients had a significantly higher rate of decompensating events. And as such, these biomarkers that we use as diagnostic biomarkers can also be used repeatedly to predict clinical outcomes and clinical events.
The same is actually true not only for liver decompensation but also for overall mortality over the follow-up of 150 months here in this study, and you can see that any change or increase was significantly associated with an overall mortality increase in these patients. Now, if we test and establish a diagnosis for these patients, we want to also impact on that prognosis using medical therapy.
And this is a study here that I’d like to share with you that has nicely shown that the use of non-selective beta blockers can actually improve the outcome in patients with clinically significant portal hypertension, even irrespective of the presence of varices and prevent the first decompensation. It’s a study that’s been published in the Lancet in 2019, therefore high-quality research.
And you can see here two groups being compared, a placebo group and a beta blocker group, shown on this graph as the cumulative incidence for the primary endpoint, death or decompensation. The primary endpoint was actually death and the placebo-treated group was significantly worse compared to the beta blocker-treated group, showing a survival benefit for beta blockers. Importantly, carvedilol was significantly more successful in reducing events here.
Having talked about a variety of non-invasive tests, I’d like to expand that group of tests by something that has been around for some time, but has seen some refinement more recently. I’m talking about spleen stiffness: we’re using liver stiffness evaluations to actually rule in or rule out compensated advanced chronic liver disease, and in the gray zone as it was present for my patients, liver stiffness below 20, and platelets above 150.
The question is, can we use spleen stiffness to assess and risk stratify these patients? In addition, and this is a study that was published by Colechia already in 2018 in the Journal of Hepatology, where you can see, yes, actually even in the stage below 20 kilopascals liver stiffness. There is an increase of spleen stiffness, and if that can be detected, the cutoff that was published in this paper – spleen stiffness measurement above 46 kilopascals – EGD was still required because these patients started to have clinically significant portal hypertension and varices were present.
But if you can rule out patients below the spleen stiffness value of 46 kilopascals, you can actually spare screening endoscopies. And in that study, this added significantly, on top of the Baveno criteria, in risk stratifying patients.
And as clinicians, I think we’re well aware of the tight connection of liver and spleen, even in the pre-cirrhotic area, that sweet spot where NITs might have difficulties to identify patients, changes in spleen stiffness can be used to further risk stratify patients. And we’ve actually used that technology more recently, and this is the result from the patient I showed you.
Again, this was a patient if you remember correctly with 11.1 kilopascals on liver stiffness and normal platelets, her spleen stiffness was 34.3 falling into a low category of spleen stiffness area and, as such, she would not require screening endoscopy to rule out varices.
And this has also been documented in the Baveno criteria. You can see here that spleen stiffness measurements can be used to rule in or rule out clinically significant portal hypertension.
However, I think further research is needed to verify the best cutoffs and, importantly, the exam that should be used to assess spleen stiffness is actually the 100 Hertz exam. Be reminded that liver exams are being performed at 50 Hertz.
So what else is there? I talked a lot about elastography. I think useful tools we’re seeing from clinical research consortia are direct fibrosis markers. ELF has been approved as a predictive biomarker, and the Litmus and Nimble Consortium evaluated ELF and its predictiveness to rule in cirrhosis. This is on the left-hand side, a study that was presented by Dr. Arun Sanyal at AASLD 2021 with a unit index of 10.1 to actually rule in cirrhosis in their patients.
And if you look into the published literature and the approved test, a score of above 9.8 predicts advanced fibrosis and a score above 11.3 is commonly used according to the manufacturer, to predict cirrhosis. Therefore, ELF is probably one of the tests that can be used if you have access to it and prefer lab-based testing.
And a second direct fibrosis marker that’s very similar to ELF, not a compound, but a solely used collagen marker, procollagen C3 or pro C3. This has been published, among others, that you can use pro C3 to risk stratify patients according to liver histology when you compare this.
So, coming back to my case, I think I’ve shown you the applicability of a number of non-invasive tests in that patient. It’s not always easy to decide if it’s cirrhosis or not. We did a liver biopsy in this patient, and you can see here the H&E stain with a liver that does contain fat, ballooning, and inflammation and also advanced bridging, potentially complete nodules.
This is the fibrosis stain. I’m not a histopathologist, so I’m not going to comment on the final diagnosis. But you do appreciate that these are at least almost complete septa. Therefore, again, a patient with compensated advanced chronic liver disease, potentially cirrhosis. Something I like to do in my clinical practice pattern is actually look at the liver laparoscopically and this is one very unique and simple way to obtain a liver biopsy, also, potentially, with repeated passes. And you can see that the liver surface of this patient starts to show some nodularity supporting the concept that this is an early cirrhosis or imminent cirrhosis in that case.
So with that, I’d like to summarize. I talked today about non-invasive assessment of compensated advanced chronic liver disease and portal hypertension. I think it’s important that we identify these patients today non-invasively – liver biopsy is not always needed. I’d like to add that the later liver biopsy we re-performed in the patient I presented you was within the scope of a clinical trial and thus had a different indication setting compared to clinical practice.
It’s important to identify these patients because they have higher morbidity and mortality and more liver-related events. There are ways to manage them, even if you’re not able to treat the underlying disease – it could be for non-alcoholic fatty liver disease today.
The use of non-selective beta blockers is beneficial for these patients. And of course, I think this is something I’d like to remind myself and you as colleagues; we’re talking about disease continuum and when we take a snapshot using liver biopsy or an NIT, we always get in just one point of the picture. It’s something where we have to monitor these patients repeatedly, do a clinical risk prediction and of course, follow them up using NITs repeatedly.
And the one study I showed you is that if you use those NITs repeatedly, you can actually say something about the prognosis based on the change of those NITs over time. And with that, we’re at the end of my presentation, I’d like to thank you for your attention. Hope you enjoyed this program. I’m open for any feedback you might have based on that program. Thank you so much.




