Introduction about Professor Laurent Castera
Good afternoon, my name is Laurent Castera, a hepatology specialist based in Paris, hospital Beaujon and professor of hepatology at the University of Paris Cité. So my tasks within the next 20 minutes will be to deal with our integrated NASH assessment and management into the care of diabetic patients.
Overview of How to Integrate NASH Assessment and Management Into the Care of Diabetic Patient
Here’s the overview of my lecture. I will cover multiple areas:
Table of Contents
- Overview Diabetic Patient Profile for this Case
- Liver is Not in the Radar of Most Diabetologists
- High Prevalence of NAFLD in T2DM patients & Limitations
- Introducing the QUID NASH Program
- High Prevalence of NASH & F3F4 in T2DM Patients Despite Mild Liver Tests Abnormalities
- Prevalence of NASH & F≥2 in T2DM Patients
- Screening T2DM Patients for NAFLD: Conflicting Recommendations
- T2DM = 2-fold Increased Risk of Severe Liver Outcomes
- F3F4 is the Major Prognostic Driver in NAFLD
- Creating Pathways Between Primary Care and Liver Clinics
- EASL Algorithm for Detecting F3F4 in NAFLD
- AACE/AASLD Recommendations for Detecting F3F4 in NAFLD
- AGA Recommendations for Detecting F3F4 in NAFLD
- Using EASL Algorithm in T2D Patients in Primary Care
- Suboptimal Performances of EASL Algorithm for Detecting F3F4 in T2DM Patients
- Factors Associated with F3F4 with Probability
- Why Collaboration Between Diabetologists and Hepatologists is Critical for our Patients – Summary
Overview Diabetic Patient Profile for this Case
So Mr N. is 55 years old and has type 2 diabetes with an abnormal liver test. It is a typical patient who we see in our patient clinic. Type 2 diabetes is treated with metformin, and dyslipidemia is treated with a statin.

His weight is 100kg and is 170 centimetres in height, so his BMI is 41.3kg/m2, waist circumference (WC) is 120 centimetres. He has no signs of chronic liver disease. He has mild abnormal liver tests, as I mentioned already, HD 52 units, ALT 37 IU, GGT 120 normal bilirubin level. Platelet count is normal at 150, PT also, 95%.
A1C is 8%, HDL cholesterol 0.30 grams per litre, and triglyceride 2.2 grams per litre. Ferritin is elevated above 800, but the saturation of transferrin is normal, and he has a bright liver on ultrasound.
liver is Not in the Radar of Most Diabetologists
So, in fact, in the current practice of most endocrinologists, the diabetic liver is not on their radar. They’re used to check for microvascular complications, including the eye, kidney, and nerves and macrovascular complications, including the brain, stroke, for instance, myocardial infarction or vascular disease, but the liver is not on their radar.
High Prevalence of NAFLD in T2DM patients & Limitations
However, the prevalence of NAFLD in type 2 diabetes patients has been shown to be as high as 55% in this meta-analysis from Dubai University. 80 studies, almost 50,000 patients with type 2 diabetes, and this is twice higher than would be expected in the general population. Now, if you look at the prevalence of severity of NAFLD advanced fibrosis, you can see that the number of studies dramatically decreased because of liver biopsy.
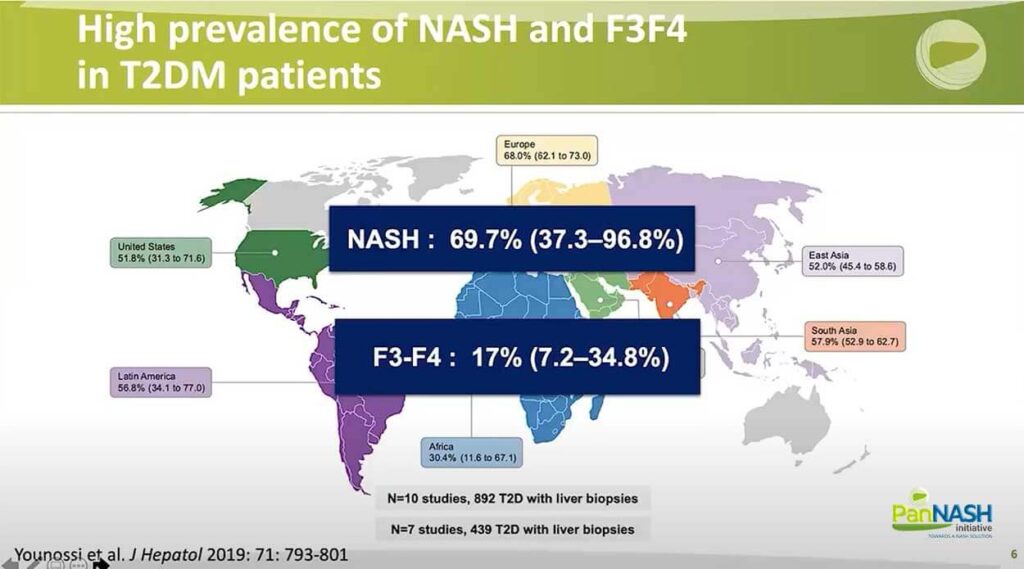
Still, the prevalence of NASH is very high – close to 70%, and the estimated prevalence of advanced tribe resists is 17%, But this is based on seven studies only with less than 500 patients with liver biopsies. As you can see, the confidence interval is very broad.
So there are many limitations in a population-based epidemiological study are weakened by the absence of liver histopathology. On the other hand, studies based on liver pathology are limited by small sample size, spectrum bias, and a variety of case definitions and are clear indications for liver biopsy. That’s the real prevalence of NASH, and advanced fibrosis in patients with type 2 diabetes remains poorly known so far.
Introducing the QUID NASH Program
So I would like to introduce the QUID NASH program. The objective of this study was to properly assess the prevalence of and features associated with histologically proven NASH and advanced fibrosis as possible indicators for further liberal assessment. So we have enrolled 713 type 2 diabetes patients with NAFLD seen in 4 outpatient clinics, of whom 330 underwent a liver biopsy.
I want to stress that the indications for liver biopsy in this court first were standardized, second, not based on pre-selection with non-invasive tests, which is quite unique. The indication was based on an abnormal liver test that is personally elevated ALT above 20 International units, in-unit females, and 30 international units in males and, of course, no other go for liver disease.
High Prevalence of NASH & F3F4 in T2DM Patients Despite Mild Liver Tests Abnormalities
When we look at the result, you can see on the left of the slide the prevalence of NASH was 58%. So in keeping with what has been reported before, but the prevalence of fibrosis, you can see the different stages going from F0 to F4, and to make a long story short, the prevalence of advanced fibrosis, the most important endpoint, because this is related with a liver-related event, liver mortality. And all-cause mortality was above all 38%, including 10% with cirrhosis. This is an all-comer population seen in diabetics clinics case in France.
Prevalence of NASH & F≥2 in T2DM Patients
Regarding the prevalence of at-risk NASH and significant fibrosis, this was 40% in this population. And this patient will qualify for pharmacologic treatment when the drug is approved.
Screening T2DM Patients for NAFLD: Conflicting Recommendations
So, what about screening diabetic patients for NAFLD? The recommendations in the literature, as you can see, are conflicting.
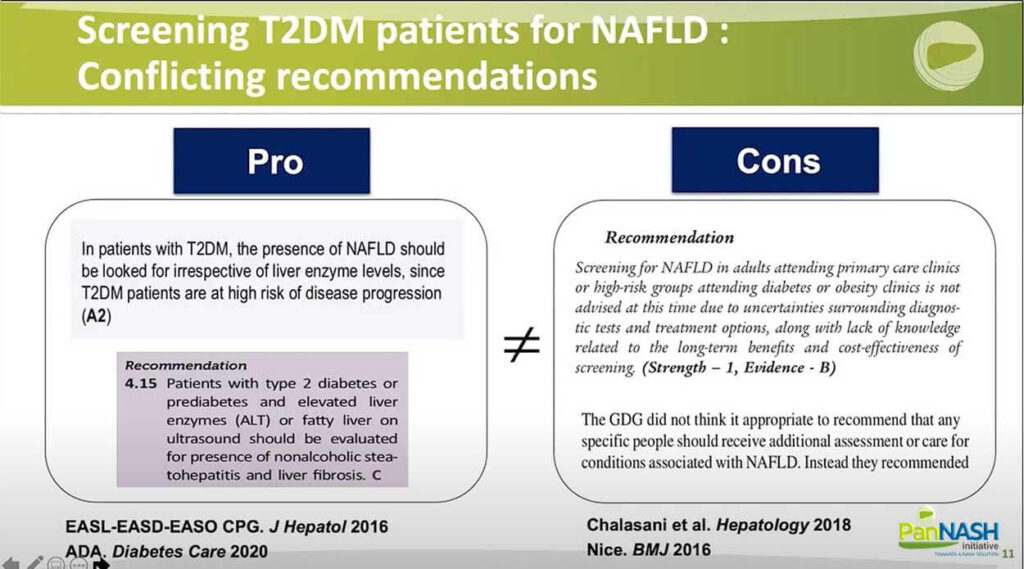
Some as joined the guideline easily published now six years ago that is in favour of screening systematically type 2 diabetic patients. And also, more recently, the ADA guideline in 2020 provided similar recommendations. On the other hand, the latest SLD guideline was against systematic screening as the NASH guideline in the UK.
T2DM = 2-fold Increased Risk of Severe Liver Outcomes
However, if you look at this methanolysis, published two years ago, a patient with type 2 diabetes is up to a twofold increased risk of several outcomes.
F3F4 is the Major Prognostic Driver in NAFLD
Also, as I mentioned before, advanced fibrosis is a meter prognostic driver enoughly; both for liver-related mortality and all causes of mortality. This is the latest and the largest study to date, prospective from the NASH CRN with a million follow-ups over four years.
Creating Pathways Between Primary Care and Liver Clinics
So clearly, if we want to screen diabetic patients, we need to create a pathway between primary care and liver clinic. In the general population, of course, the risk factor for NAFLD are well known: apart from type 2 diabetes, of course, age is about 40 years, obesity and more generally features of the metabolic syndrome.
Most of these patients are seen in primary care, where the prevalence of advanced fibrosis is much lower than in the liver clinic, below 5%, probably around 2% to 3%. For this, you need to start with a very simple test, easily or widely accessible and very cheap. The best tests that qualify are serum biomarkers and among serum biomarkers, FIB-4, which is composed of age transaminase and platelet count.
And if FIB-4 is below 1.3, the likelihood of having advanced fibrosis is very low, and this patient does not need to be referred to a specialist. On the other hand, if FIB-4 is above 1.3, they should undergo a second examination: the most popular one is transient elastography, better known as fibroscan; and so far, this is mostly available in liver clinics.
But what about the diabetes clinic? Prevalence had shown that it is probably higher than anticipated, and it’s in between primary care and liver clinic, and this patient should be screened systematically, and pathways need to be created.
EASL Algorithm for Detecting F3F4 in NAFLD
This is the EASL algorithm that has been proposed for detecting advanced fibrosis in NAFLD. So again, as I mentioned, FIB-4 and if you are lower than 1.3, there is no need for referral lifestyle modifications, and you can retest, of course. If you’re above, you should undergo a second exam; the first one is liver stiffness and the cut-off 8kPa, and you might use a second test, the patented serum tests and combine them.
When of course, there is concordance there, likely would of advanced fibrosis is very high, not even considering a liver biopsy. In other cases, you might consider a liver biopsy.
AACE/AASLD Recommendations for Detecting F3F4 in NAFLD
The interesting thing is that these guidelines either were published in September 2021, and since you can see many other guidelines, there are guidelines from Cusi K from the endocrinologists published this year, and they go in the same direction.
AGA Recommendations for Detecting F3F4 in NAFLD
The only difference is the dual cutoff strategy instead of a single cutoff, but you can see that the field is aligning, and we also have this recommendation publishing gastroenterology from AGA that use basically the same pathway fit for the liver stiffness.
So back to our patients, you can see the FIB- is above 1.3, CAP is suggestive of steatosis, and liver stiffness is elevated above 12 kPa.
Using EASL Algorithm in T2D Patients in Primary Care
This is a paper from the group of T. showing the result of the EASL algorithm in type 2 diabetic patients seen in primary care. And I started with the FIB-4, as you can see, and less than 20% of the patients had FIB-4 above 1.3, but 25 are not referred for transient elastography and out of 86, only 58; so 12% of the total on the transient elastography.
The majority, as you can see, is around 40% at living stiffness below 8 kPa. But as for the other, it fails in five cases, and the other range between 8 and 15 or above 15. At the end of the day, you can see only 20 patients, so less than 5% had a new diagnosis of advanced liver disease following specialist review. So this algorithm does not seem to be perfectly suited for type 2 diabetes patients.
Suboptimal Performances of EASL Algorithm for Detecting F3F4 in T2DM Patients
This is our experience in Quid NASH score; I’ve already presented using EASL algorithms; you can see that you end up with force negative for advanced fibrosis in 27% of cases. And if you perform a fibroscan in patients under 1.3, again, you end up with false positives in a little bit more than 30% of cases. So again, suggesting that this algorithm is not optimal in type 2 diabetes patients.
One of the issues is that the transient electrography availability is limited in most diabetes clinics and also warns us regarding NITs is usually poor among diabetologists.
Finally, very few patients with NAFLD are currently referred to a hepatologist for further assessments, and opportunities for early intervention are missed.
Factors Associated with F3F4 with Probability
So in order to address this limitation, the fact that most diabetes clinics currently do not have available or accessibility to fibroscan, we designed a very simple score based on multivariate analysis in our population to be about identified patients with advanced fibrosis, which should be referred to a hepatologist. So this score, as you can see, includes FIB-4, so H transaminase that leads count and metabolic parameters where circumference HDL-cholesterol and GGT.
You can see that the AUROC of this score was 0.77 in our population. Now, if we go back to our patient, waist circumference (WC) was 120 centimetres; you can see transaminase, platelet count and HDL-C cholesterol.
So you can go online with this course, and then you enter the age, the different other parameters, as I mentioned in our patients, and you can see that in this patient, the risk for having advanced fibrosis disease was 90%, so that is very high and this patient should be referred to a hepatologist.
Actually, this is what has been done – a liver biopsy has been performed. As you can see on this slide, he has cirrhosis and a subscore of S2A3F4. It’s a NAS of 4, so he would be a candidate for pharmacological treatment once the drug is approved.
Why Collaboration Between Diabetologists and Hepatologists is Critical for our Patients – Summary
So just to conclude. I hope I have convinced you that close collaboration between a diabetologist and a hepatologist is critical for patients in terms of management but also in the theme of awareness. Thank you very much for your attention.




