INTRODUCTION to PROFESSOR Steven Harrison
Professor Steven Harrison is a renowned expert in hepatology, holding prominent positions such as:
- Visiting Professor of Hepatology at the Radcliffe Department of Medicine, University of Oxford
- Medical Director of Pinnacle Clinical Research
- President of Summit Clinical Research
With his wealth of knowledge and experience, Professor Harrison is honored to discuss the latest non-alcoholic steatohepatitis- NASH treatment advancements in 2022.
OVERVIEW OF NASH Treatment in 2022 Current and Future Therapies for Non-Alcoholic Steatohepatitis
Here’s the overview of the lecture that will cover multiple areas:
Table of Contents
- NASH: Potential Therapeutic Targets
- Targeting NASH: Exploring Therapeutic Approaches
- Agents in Phase 2 Development-Route of Administration
- Agents in Phase 3 Development
- NASH Development Landscape
- Both NASH Resolution and Fibrosis Improvement
- Advancements in NASH Cirrhosis Studies
- The Future of NASH Therapeutics Ongoing Phase 3 Trials
- Key Insights from Past Failures: An Essential Part of the Drug Development Journey
- Agents may have different profiles of histopathologic effects (fibrosis, NASH resolution) vs Extrahepatic Effects
- First NASH Drugs in Development
- Categorizing NASH Therapeutics by Mechanism of Action (MOA)
- Future Therapeutic Considerations for Precision Medicine
NASH: Potential Therapeutic Targets
To comprehend the current state of NASH therapy, examining the disease’s pathogenesis involves multiple pathways leading to steatohepatitis development in patients is crucial.
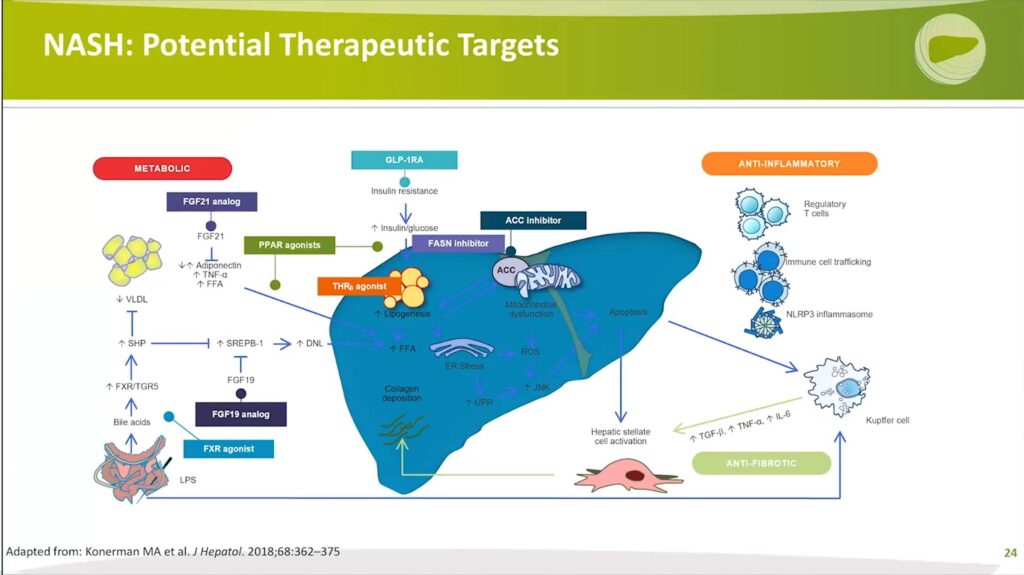
Three primary ways that NASH presents in patients include:
1. Insulin resistance: All NASH patients exhibit insulin resistance and/or have diabetes. What happens with insulin resistance is adipocytes become dysfunctional, releasing free fatty acids that are very toxic to the liver. These fatty acids are taken up in the portal vein and delivered to the liver, where the liver has a couple of options; those toxic-free fatty acids can be:
- Esterified to triglyceride and stored as fat
- Burned through beta-oxidation within the mitochondria
- Repackaged and shipped out of the liver in the form of very low-density lipoprotein
2. Upregulated de novo lipogenesis: Additionally, in patients with underlying non-alcoholic fatty liver disease, NAFLD and NASH show a disproportionate amount of upregulated de novo lipogenesis, providing another opportunity for target therapeutics.
3. Additional contributing factors: As livers become saturated with toxic-free fatty acids, mitochondrial dysfunction begins to take place. Endoplasmic reticulum stress and an increase in the unfolded protein response ensue, driving pro-inflammatory mediators accentuated and the development of reactive oxygen species.
This leads to apoptosis and subsequent activation of hepatic stellate cells. There’s also an overlay of dysfunctional regulation with the immune cell pathways, which can also contribute. In addition, the microbiome may be playing a role here as well, and genetic influences such as HSD17B13 and PNPLA3 may also be contributing to the underlying pathogenesis of the disease.
These various factors offer numerous opportunities for drug targets, many listed here, which can be categorized as metabolic, anti-inflammatory, and anti-fibrotic.
Most therapies being developed today for NASH target metabolic processes, as illustrated via FXR agonism, FGF19, FGF 21 agonists, PPAR Agonists, thyroid hormone receptor beta (TRβ) agonists, GLP-1 Receptor Agonists, FASN inhibitors and Acetyl-CoA Carboxylase inhibitors.
Nonetheless, it should be noted that other pathways, such as those targeting anti-inflammatory and direct anti-fibrotic therapeutics, are also being explored, albeit in a smaller number of studies.
Ultimately, Professor Steven Harrison will present you the treatment of NASH using combination therapy, which targets multiple pathways simultaneously. This approach holds the potential to provide a more comprehensive and effective treatment for patients suffering from this complex and multifaceted disease.
Targeting NASH: Exploring Therapeutic Approaches
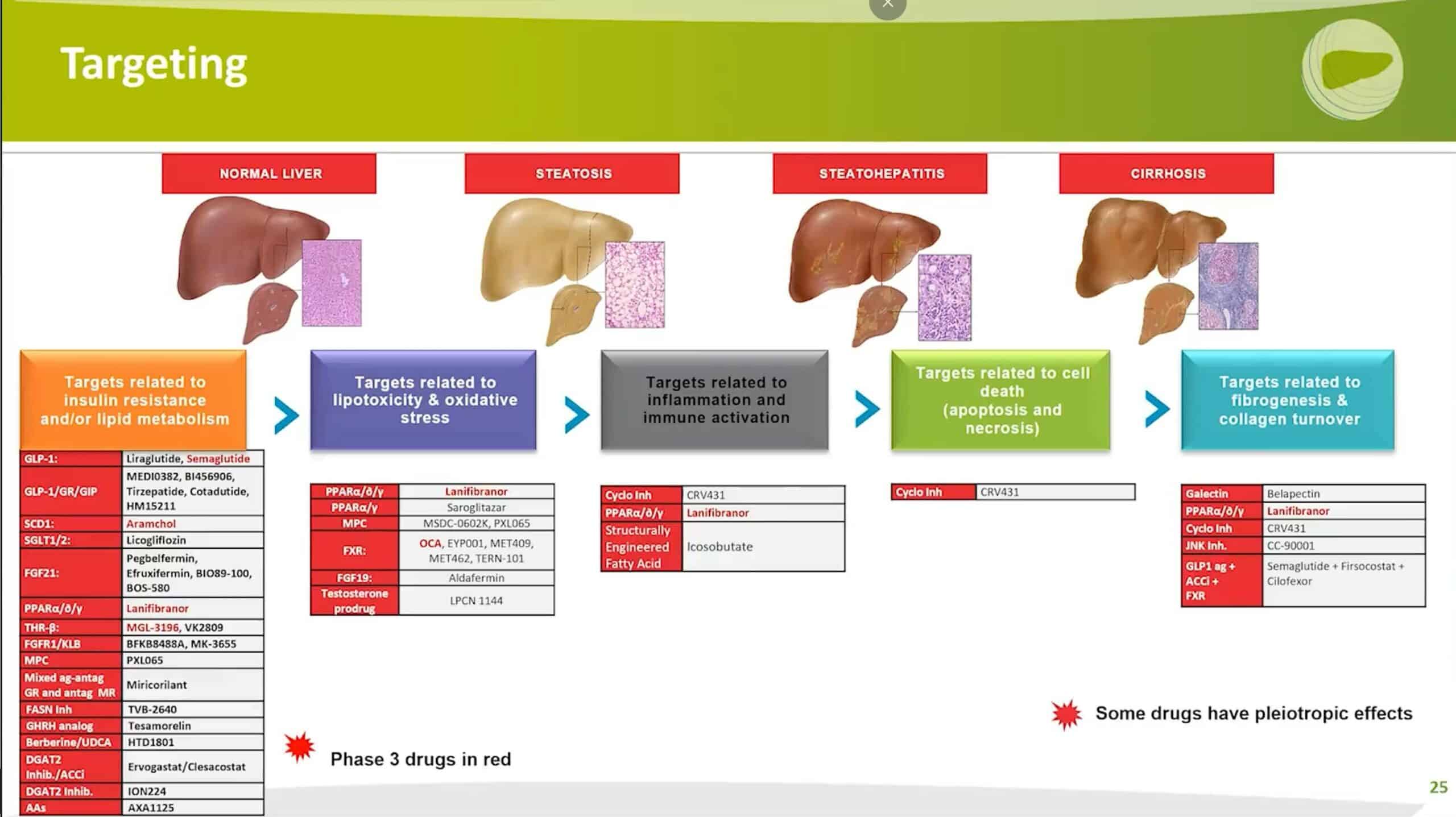
Professor Steven Harrison delves deeper into NASH treatment options by classifying drug therapies into five distinct categories:
- Targets related to insulin resistance and/or lipid metabolism;
- Targets related to lipotoxicity and oxidative stress;
- Targets related to inflammation and immune cell activation;
- Direct targeting of stellate cells;
- Targeting cells that are undergoing apoptosis or necrosis;
Professor Harrison’s presentation highlights all treatments currently in development, with a special emphasis in red for those in Phase 3.
As evident, the majority of treatments fall within the first two categories, with some development done on fibrosis and a few with immune cell activation and targets related to cell death. It is important to note that some of these drugs have pleiotropic effects and may appear in multiple categories.

Focusing on drugs in Phase 2, Professor Harrison further divides them into Phase 2A and Phase 2B. Phase 2A trials are typically shorter in duration and non-invasive, meaning they do not involve histopathology. A wide range of drugs is currently in development for this phase.
In contrast, Phase 2B trials generally require paired liver biopsies—a biopsy for entry and another for exit. These trials are also larger in scope and longer in duration.
Prof. Harrison highlights drugs currently in Phase 2B with a shaded green box. Using the arrow, these drugs have either completed enrollment or have reported their results.
As evidenced in his presentation, a tremendous amount of activity is underway in the field of NASH treatment. The year 2022 promises to deliver numerous reports on these drugs, and there is hope for positive results that will advance treatment options for patients with NASH.
Agents in Phase 2 Development-Route of Administration
Professor Steven Harrison classifies Phase 2 treatments according to their route of administration, dividing them into oral agents and injectable or infusion therapies.
Focusing on injectable and infusion therapies, Professor Harrison explains that hormones must be administered through injections. There is no oral therapy for that:, as seen with FGF19 and FGF21 analogs. Aldafermin, the only FGF19 analog, is currently in a Phase 2B trial for well-compensated cirrhosis.
Multiple drugs and multiple companies are studying FGF21 Agonists, with many trials well underway. It is anticipated that Akero® will reveal their 24-week paired liver biopsy study later this year.
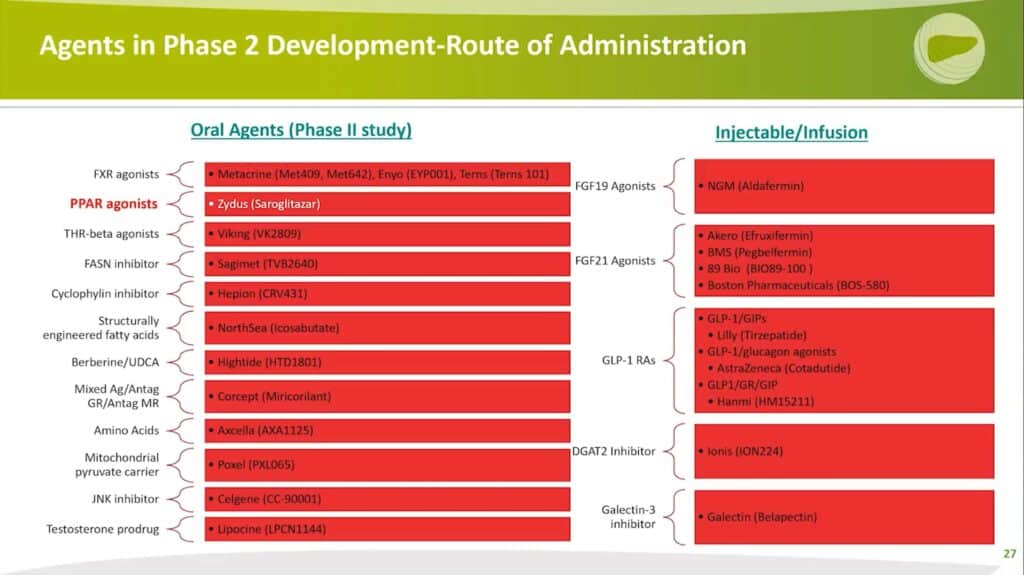
GLP-1 receptor agonists include not only standalone GLP-1 therapies but also combinations with GIPs (e.g., Tirzepatide) or glucagon agonists (e.g., Cotadutide or Penvudutide) and triple agonists by Hanmi.
D-GAT2 inhibitors use antisense technology and work with de novo lipogenesis (DNL to block de novo lipogenesis).
Lastly, Galectin-3 is the only drug in development aiming to prevent disease progression: it is an infusion administered every two weeks, currently in an adaptive Phase 2B/3 study. The treatment focuses on patients with well-compensated cirrhosis without esophageal varices, aiming to prevent the progression of varix to varices.
All the agents mentioned by Professor Harrison target improvements in underlying histopathology or non-invasive technology, offering promising advancements in NASH treatment.
Agents in Phase 3 Development
Now, let’s look at the drugs in Phase 3; there are currently five: However, one of them, Aramchol, is currently on hold and will not be discussed further.
The four active Phase 3 agents in development are as follows:
- FXR Agonists: Obeticholic Acid
- pan-PPAR Agonists: Lanifibranor
- A TRβ Agonist: Resmetirom
- GLDP-1 Receptor Agonist: Semaglutide

These four drugs represent the latest advancements in NASH treatment and showcase the potential for new therapeutic options for patients suffering from this complex disease.
Nash Development Landscape
1. Fibrosis Improvement
Professor Steven Harrison discusses the drugs that have shown evidence of benefiting patients from a fibrosis perspective. The following drugs are listed across the top:
- Efruxifermin
- Lanifibranor
- Aldafermin,
- Resmetirom
- Seladelpar
- Aramchol
- Semaglutide
- Ocaliva®
Additionally, the dosing duration is indicated above each drug.
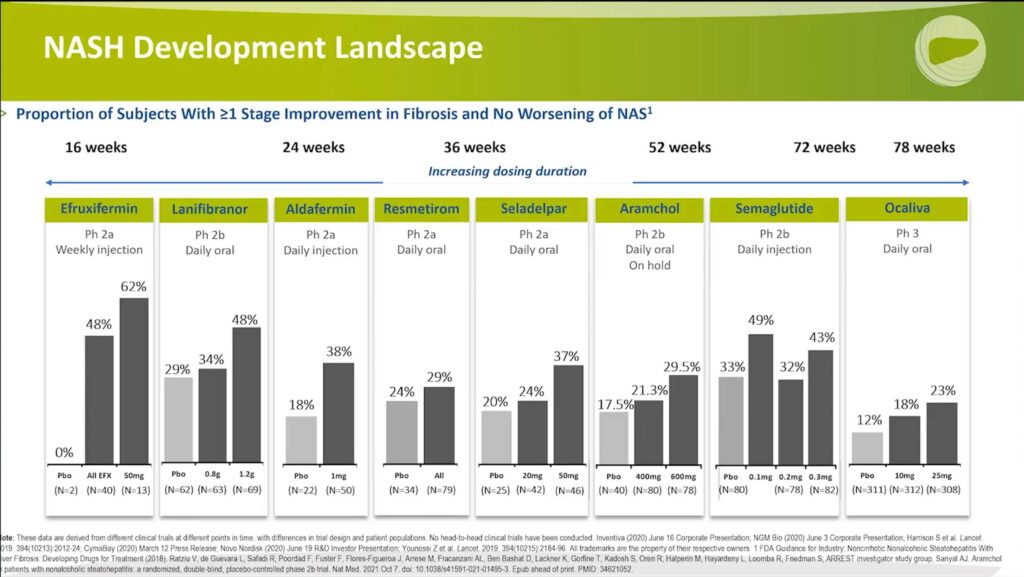
These bar graphs represent the proportion of subjects who experienced at least one stage of improvement in fibrosis without worsening of the non-alcoholic fatty liver disease activity score (NAS). This analysis highlights the potential of these drugs in improving fibrosis, a crucial aspect of NASH treatment.
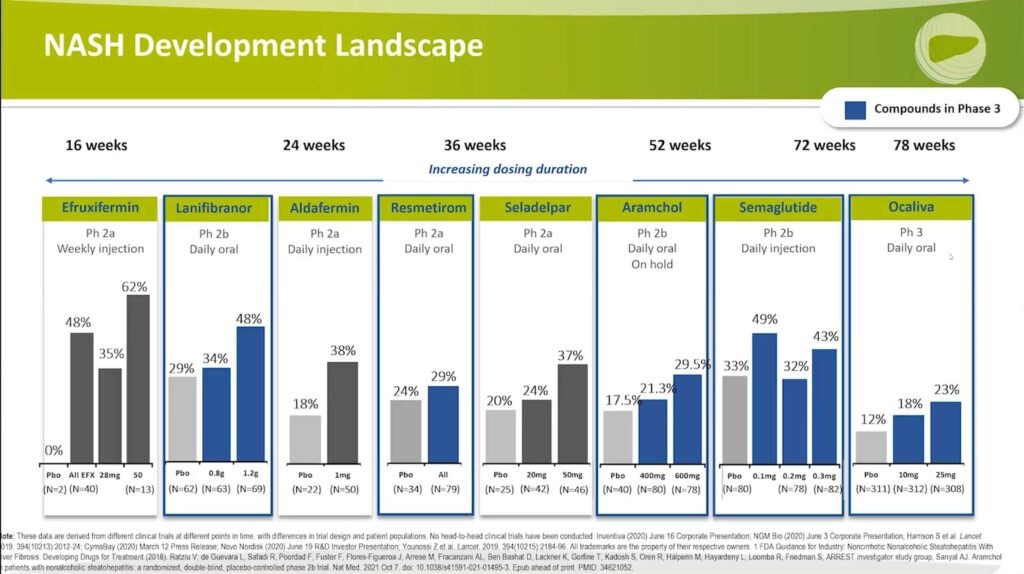
1.1 Compounds in Phase 2
First, let’s examine the drugs that are in Phase 2, which are highlighted in green:
Aldafermin: This FGF19 analog demonstrated a 20% point treatment effect delta with a one-milligram dose versus placebo when administered for 24 weeks.
Efruxifermin: This drug was dosed for 16 weeks and showed a 62% improvement in fibrosis with the high-dose group and 48% for all comers, compared to placebo with zero. It is important to note the study design – patients only underwent liver biopsy if they had improvement in the non-invasive test, particularly MRI PDFF. As a result, only a small number of patients in the placebo group underwent a biopsy. Despite the small sample size, this drug’s potential impact on fibrosis is evident.
Seladelpar: A PPAR Delta Agonist and oral therapy, Seladelpar was studied for 36 weeks. The high-dose group showed a 37% response on fibrosis, with the placebo group at 20%. Although this result was not statistically significant, it was a post hoc analysis and not powered for that endpoint. The drug’s potential to improve fibrosis is still notable.
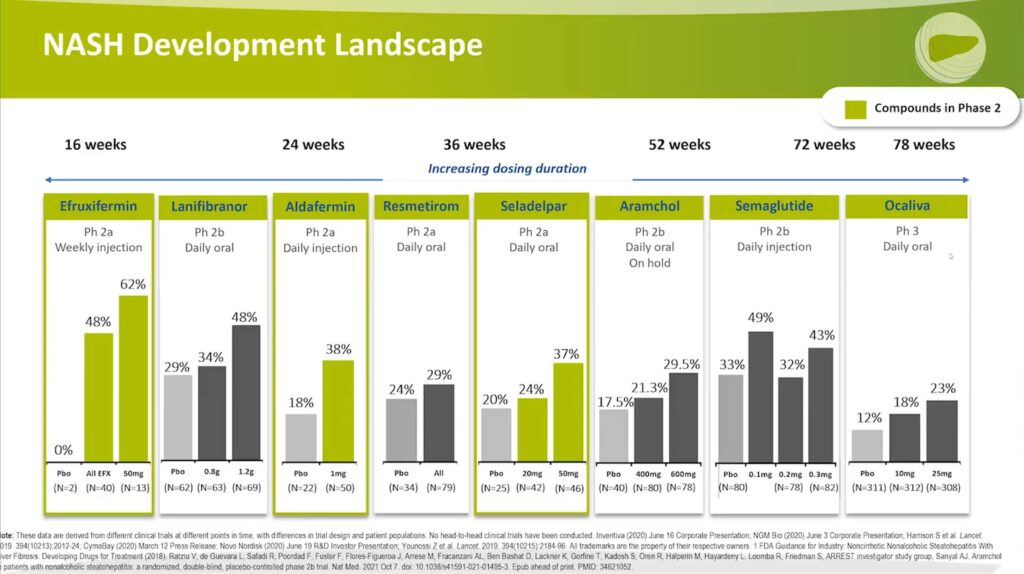
1.2 Compounds in Phase 3
The discussion focuses on the results from Phase 2 trials for compounds that have advanced to Phase 3 development:
- Lanifibranor: This drug exhibited a 48% fibrosis improvement rate with a 1.2-gram dose compared to 29% for the placebo. The fibrosis treatment effect delta was significant.
- Resmetirom: The fibrosis improvement rate was 29% versus 24% for the placebo. However, the treatment effect delta was not significant. Consequently, Resmetirom’s Phase 3 trial focuses primarily on NASH resolution.
- Aramchol: The high-dose group demonstrated a 29.5% fibrosis improvement rate compared to 17.5% for the placebo.
- Semaglutide: The fibrosis improvement rates were between 32% and 49% with the drug versus 33% for the placebo. This result was not statistically significant.
- Ocaliva®: The fibrosis improvement rate was 23% compared to 12% for the placebo, with a significant treatment effect delta of 11%. The Phase 3 trial was the REGENERATE trial, which led to the initial filing for a new drug application. However, the FDA did not approve the drug due to concerns about the therapeutic index and associated adverse events.
Ocaliva® announced on July 7th that it plans to refile the drug based on additional data, including further safety data. More updates will follow as the refiling process begins.
2. NASH Resolution
2.1 Compounds in Phase 2
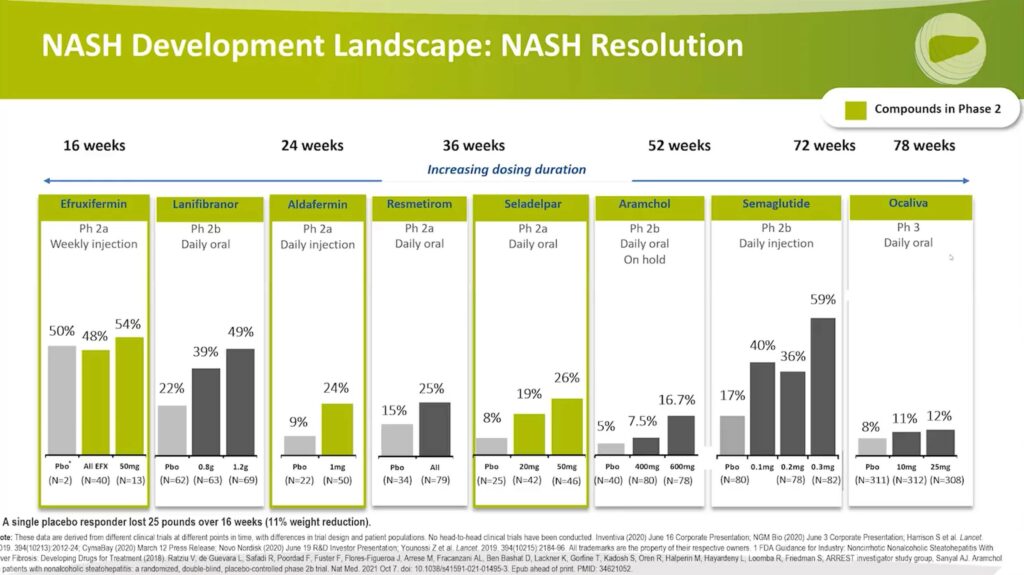
Examining NASH resolution, the same color coding is used for drugs from Phase 2.
- Efruxifermin shows a 54% to 48% response compared to the placebo at 50%. It is important to note that only two patients in the placebo group underwent a biopsy, which may affect the numbers. Further data is needed to understand better the effectiveness of this FGF-21 Agonist on NASH resolution. Additionally, the treatment duration was only 16 weeks. One patient who responded experienced a 25-pound or 11% weight reduction during the treatment period, which could explain the NASH resolution.
- Aldafermin demonstrates a 24% response compared to the placebo at 9%.
- Seladelpar exhibits a 26% response compared to the placebo at 8%.
2.2 Compounds in Phase 3
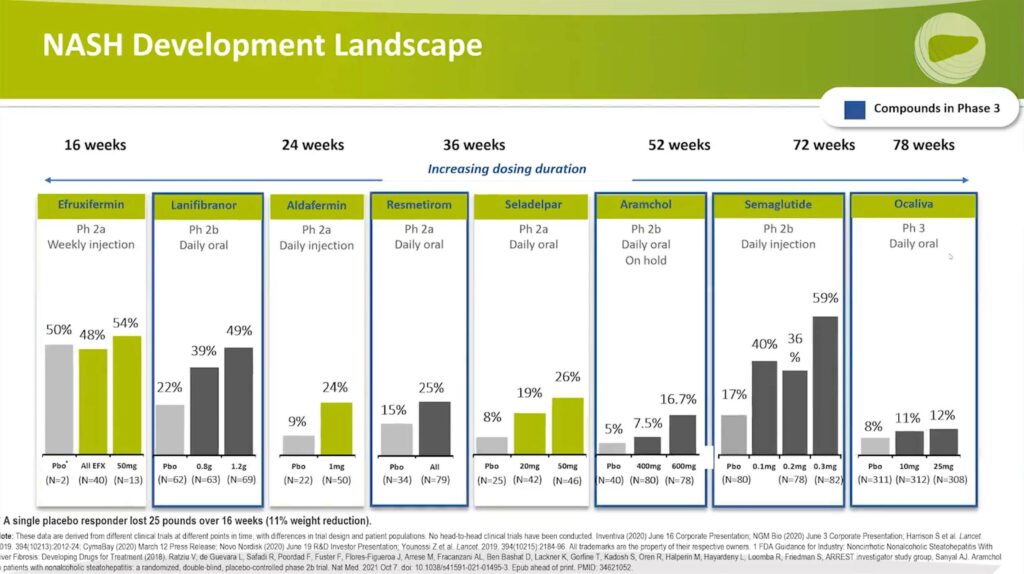
Analyzing the drugs now in Phase 3, the response rates are as follows:
- Lanifibranor shows a 49% response with the high dose compared to 22%, which was significant.
- Resmetirom exhibits a 25% response versus 15%, which was significant.
- Aramchol is currently on hold, but it demonstrated a 16.7% response with the high dose compared to 5%.
- Semaglutide has a 59% response with the 0.3-milligram daily injection dose versus 17% for the placebo.
In contrast, Phase 3 data for Ocaliva® reveals a treatment effect of 12% with the high dose compared to 8%, which was not statistically significant.
Both NASH Resolution and Fibrosis Improvement
Investigating Phase 2 Compounds
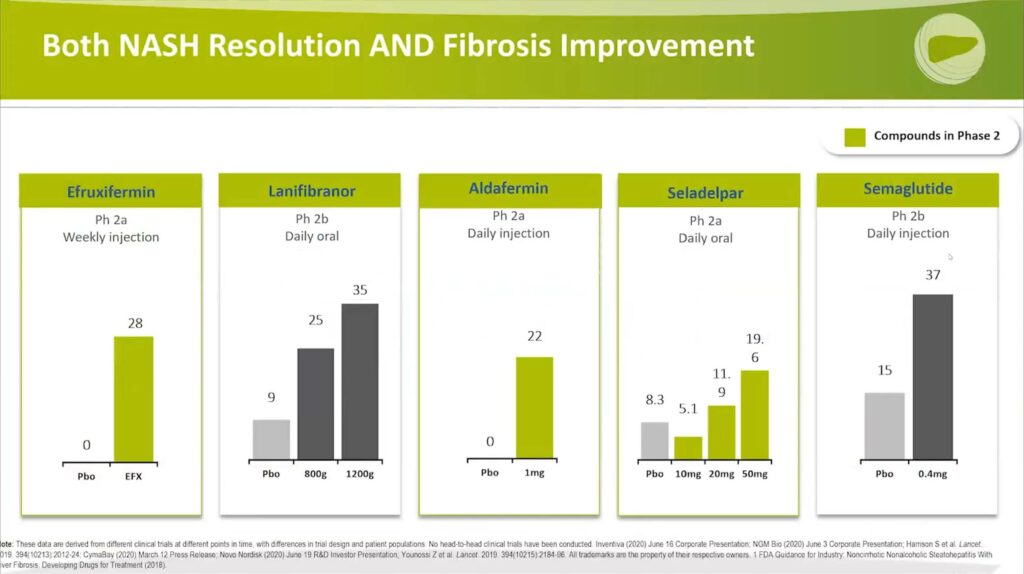
Reaching the dual objectives of NASH resolution and fibrosis improvement presents a considerable challenge. However, promising outcomes have emerged from the phase 2 trials of Efruxifermin, Aldafermin, and Seladelpar:
- Efruxifermin: presented significant results, with 28% achieving both NASH resolution and fibrosis improvement, compared to 0% in the control group.
- Aldafermin: delivered a comparable outcome, with 22% versus 0% achieving the dual targets.
- Seladelpar: indicated a promising dose-response relationship, achieving 19.6% in the high dose group compared to 8.3% in the placebo group.
What is consistently observed with this histopathologic endpoint is that it’s harder for a placebo to achieve success. Many researchers consider this an attractive target because it stabilises the placebo response rate, which can help reveal the true effectiveness of a drug in comparison.
A consistent observation across these results is the reduced likelihood of placebo achieving successful outcomes when measuring this histopathologic endpoint. For many researchers, this makes the dual-target a more desirable benchmark. While it raises the hurdle for drug performance, it helps to stabilize the placebo response rate, potentially providing a more accurate comparison of drug effectiveness.
While it’s a challenge for a drug to reach these dual targets, it’s a more effective way to uncover the truth – the ‘ground truth’. For instance, how effective is Aldafermin truly, with 22% versus 0% outcomes? And what about Seladelpar with its 19.6% versus 8.3% results? These disparities illustrate why stabilizing the placebo response rate is crucial for a valid comparison.
Exploring Phase 3 Compounds
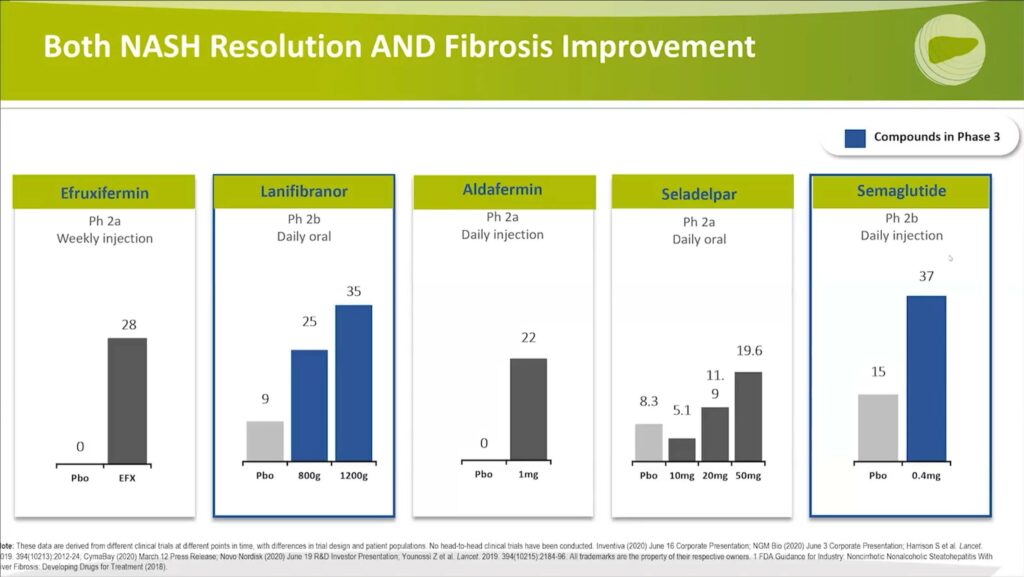
Progressing to Phase 3 compounds, Professor Harrison sheds light on the performance of Lanifibranor and Semaglutide:
- Lanifibranor shows a substantial dose-response relationship, with results of 35%, 25%, and 9%, compared to the single-digit placebo response.
- Semaglutide, on the other hand, yielded a slightly higher placebo response rate of 15%, which wasn’t observed with other compounds when investigating this dual target. However, Semaglutide also showed a higher response rate of 37%.
These clinical trial phases provide critical insights into how well these compounds are functioning against the dual targets of NASH resolution and fibrosis improvement. The stabilization of the placebo response rate remains a central factor in evaluating the true effectiveness of these potential treatments for NASH.
Advancements in NASH Cirrhosis Studies
Moving onto the cirrhosis area, Professor Harrison navigates the current state of drug development in this sphere.
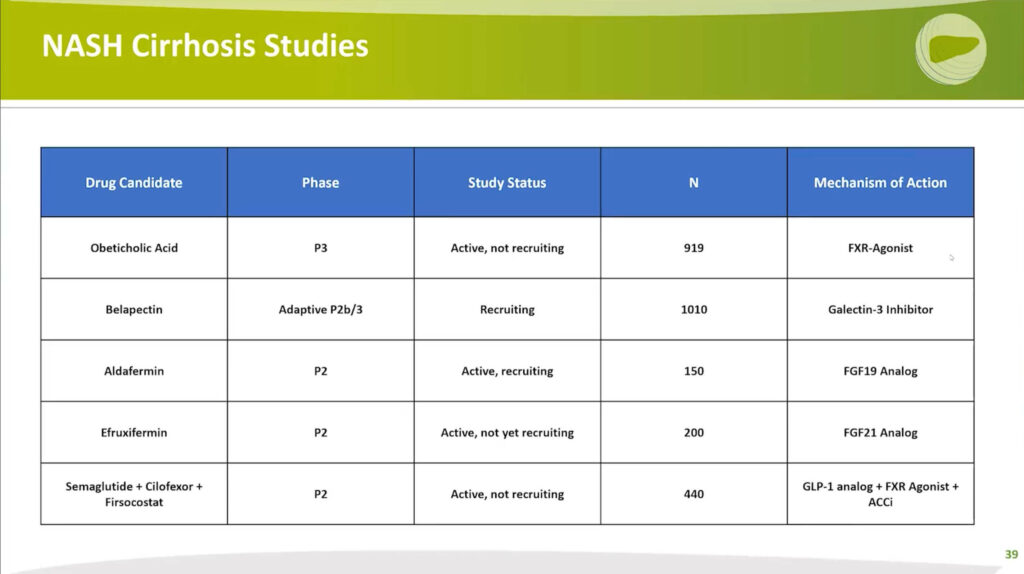
There are five ongoing active trials in Phase 2 or Phase 3 focusing on cirrhosis treatment. Here’s an overview of the key players:
- Obeticholic Acid, an FXR agonist, is under review.
- Belapectin, a Galectin-3 inhibitor previously mentioned, is currently in use. Notably, it’s an infusion administered biweekly. The compound is undergoing unique adaptive Phase 2b/3 studies, primarily focusing on preventing varices development.
- Aldafermin, an FGF19 Agonist, is in Phase 2. This trial has fully enrolled 150 patients, and the medical community is eagerly awaiting the results.
- Efruxifermin, an FGF21 Agonist, is also in Phase 2. The trial is currently in the process of recruiting participants.
- Lastly, a combination study involving Semaglutide, a GLP-1 receptor agonist, Cilofexor, an FXR agonist, and Fircostat, an acetyl-CoA carboxylase inhibitor, is now enrolling participants. This study targets the well-compensated cirrhotic population.
Overall, these ongoing clinical trials mark crucial steps forward in the development of effective treatments for NASH cirrhosis. The field keenly anticipates the outcomes of these trials, offering potential strides in advancing NASH cirrhosis management.
The Future of NASH Therapeutics Ongoing Phase 3 Trials
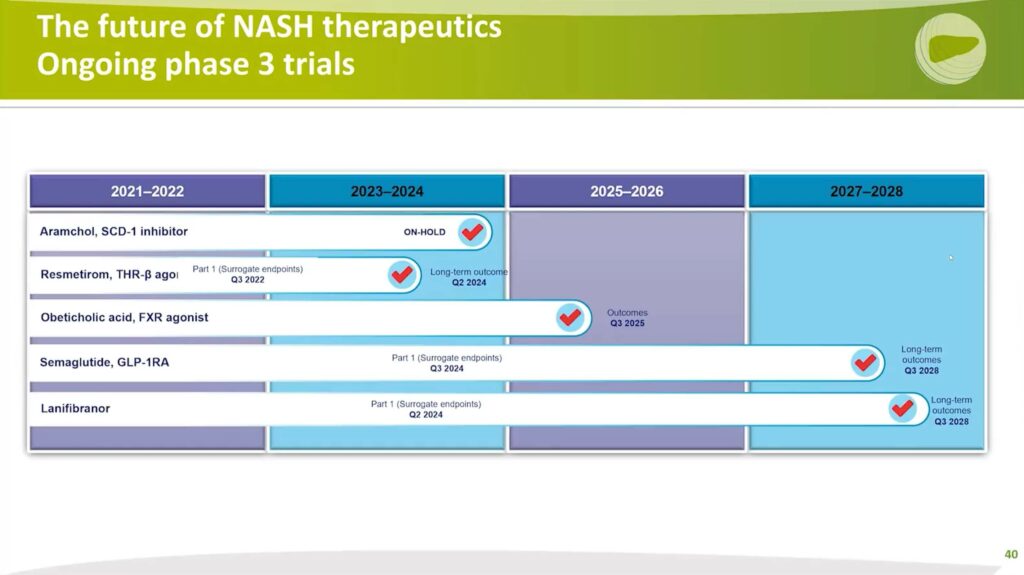
Professor Steven Harrison turns our attention to the upcoming milestones and anticipated readouts of ongoing Phase 3 trials in NASH therapeutics.
Firstly, the Aramchol trial is currently on hold. However, Resmetirom is expected to release its trial results in the fourth quarter of this year, with long-term outcomes forecasted between the second and third quarters of 2024.
Obeticholic acid was a notable mention on July 7th 2022, when a press release announced a refiling based on new data and enhanced safety endpoints. This refiling draws on safety data from a significantly larger patient pool. The study continues to progress, with an anticipated outcome slated for the third quarter of 2025. The refiling pivots on the surrogate or conditionally approvable endpoint of an 18-month liver biopsy, but the ongoing trial will still evaluate overall outcomes.
Semaglutide and Lanifibranor are still in the process of participant enrollment. The surrogate endpoint readout is projected for the third quarter of 2024 for Semaglutide, and the second quarter of 2024 for Lanifibranor.
As the trials progress, we can anticipate an evolution in our understanding and approach to NASH therapeutics. These pivotal trials will shape the future of NASH treatment, heralding a new era in the field.
Key Insights from Past Failures: An Essential Part of the Drug Development Journey
Throughout the course of his work, Professor Steven Harrison has recognized that failures in drug development are valuable learning opportunities. Indeed, there have been numerous trials that didn’t yield the anticipated success, but each failure imparts crucial lessons to researchers.
A range of drugs, including Simtuzumab, Elafibranor, Selonsertib, Cenicriviroc, and Emricasan, serves as poignant examples. Although these specific trials were unsuccessful, they still added to the collective knowledge of the field. The lessons learned from these trials serve as a guide for future drug development and underline the importance of learning from setbacks to fuel further scientific discovery.

Agents may have different profiles of histopathologic effects (fibrosis, NASH resolution) vs Extrahepatic Effects
In assessing the potential of these therapeutic agents, it’s crucial to look beyond their histopathologic impacts and consider their extrahepatic effects and tolerability profiles.
The effectiveness of drugs is usually measured against their tolerance levels, a concept known as the therapeutic index. TFor an ideal drug, the balance between efficacy and tolerability should lean heavily towards efficacy with minimal adverse effects.
To illustrate this concept more effectively, the analysis is divided into two categories: non-cirrhotic and cirrhotic, and color-codes the drugs according to their adverse events profile. A drug in the upper right-hand corner of both these categories, with high effectiveness and minimal adverse events, would be his ideal choice.
Impacts in Non-Cirrhotic NASH
In the context of non-cirrhotic NASH, Semaglutide, a GLP–1 receptor agonist, and Efruxifermin, an FGF21 agonist, show commendable extrahepatic effects. They demonstrate positive impacts on glycemic control, atherogenic lipids, and weight control, with Semaglutide contributing to weight loss and Efruxifermin maintaining a neutral to slightly downward trend with potential impacts also on blood pressure. While the histopathologic results place them in the middle tier due to small data sets, particularly for Efruxifermin, Semaglutide has shown substantial improvement in NASH, despite a lack of significant effect on fibrosis.
Lanifibranor shows slight superiority in histopathology, its extrahepatic effects are not as strong. Although it does influence glycemic control and atherogenic lipids, potential weight gain is an associated drawback.
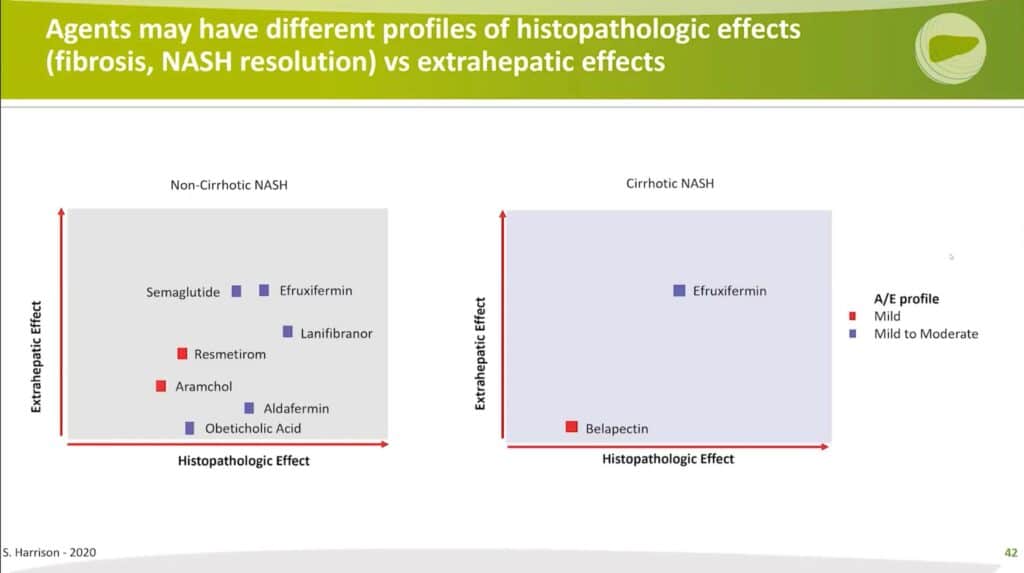
Impacts in Cirrhotic Conditions
When discussing cirrhotic conditions, available data is scarce, especially regarding liver biopsy results. Belapectin, for instance, has shown no histologic effects in Phase 2 and is currently being studied for its potential to prevent progression to varices. However, no extrahepatic benefits are currently known for Belapectin.
On the other hand, Efruxifermin, with its already established extrahepatic benefits, is limited by a lack of comprehensive histological evidence in a well-compensated cirrhotic population. A small cohort of patients studied showed that a third had resolution of cirrhosis, but the generalizability of this outcome is uncertain.
In conclusion, the evaluation of drug effectiveness for NASH should involve a multi-dimensional approach, taking into account not only the impact on the liver but also the potential extrahepatic benefits and side effects. This holistic view can lead to a more comprehensive understanding of the drug’s potential and eventual clinical application.
First NASH Drugs in Development
Currently, the field of NASH drug development could be likened to the pioneering ‘Wright Brothers’ stage. We are witnessing the initial take-off, beginning to register some response. However, our ultimate goal mirrors the precision and sophistication of the ‘F-35’ – a precision medicine ‘strike fighter’ approach. We aspire to hone in on therapeutics tailored to patients’ metabolic and genetic profiles, elevating the level of personalized medicine within the realm of NASH treatment.
Combination Therapy in NASH Treatment
The concept of combination therapy is paramount when envisioning the future of NASH treatment. Depending on the patient’s specific disease profile, including their risk tolerance, safety considerations, and the stage of liver disease progression, an ideal treatment regimen could include a combination of drugs targeting different aspects of the disease.
For instance, in cases of mild fibrosis marked by significant fat and inflammation, a therapy predominantly aimed at improving metabolic profiles could form the backbone of treatment. This could be supplemented with a second agent to slightly mitigate fibrosis.
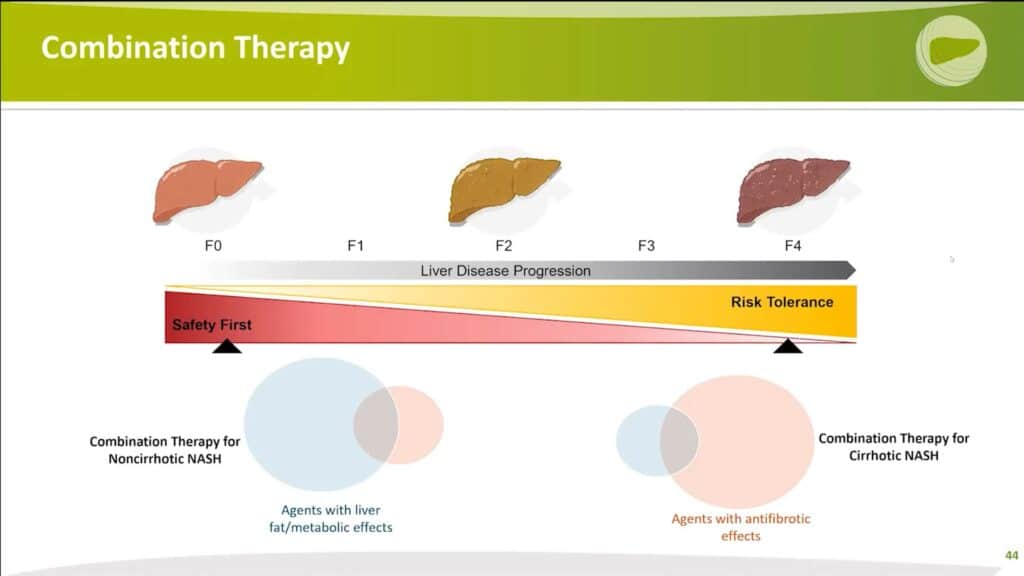
Alternatively, for patients with cirrhosis or advanced liver disease, the priority shifts to halting disease progression as quickly as possible. In such cases, an anti-fibrotic drug could be paired with a second agent offering metabolic benefits. This combined approach aims to arrest or improve the disease while concurrently preventing the formation of new scar tissue.
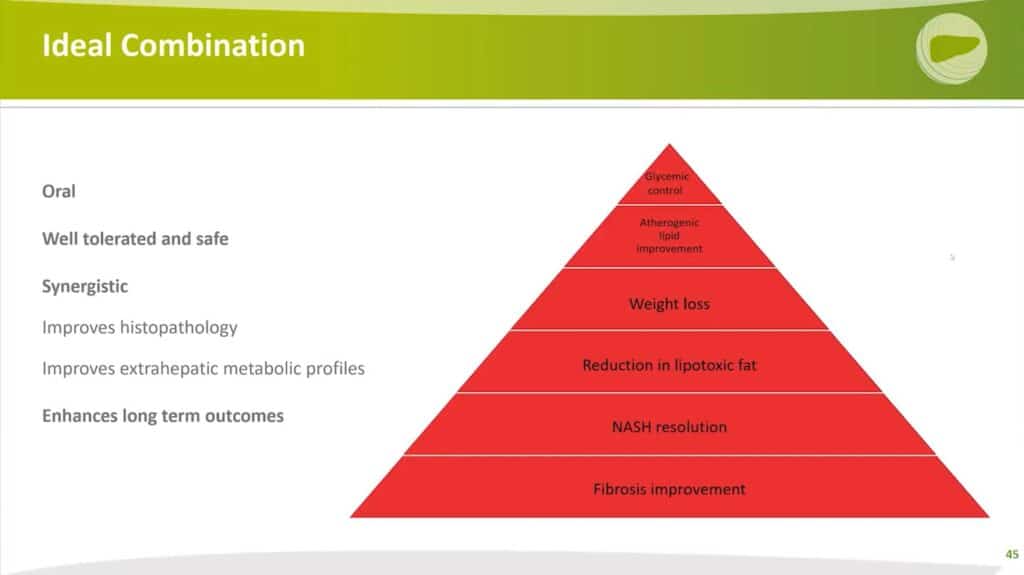
Ideal Combination Therapy
What is the ideal combination therapy in Nash Treatment?
The perfect combination therapy is one that is orally administered, well-tolerated, and safe. The ultimate goal is to consolidate this treatment into a single pill, blending therapies that work synergistically to improve both histology and extrahepatic metabolic profiles. With long-term improvement of patient outcomes in mind, the aim is to mitigate not only major adverse liver outcomes but also major adverse cardiac events.
To visualize this concept, consider the Jamaican 4×100 relay world record team from 2011. Each member of this team was a world-class sprinter in their own right, they worked well together, and each brought complementary strengths to the team. They were all in it for the long haul, similar to the ideal combination therapy for NASH.
The perfect combination therapy should be ‘world-class’ in its own right, synergistic and harmonized, offer complementary strengths, and be designed for long-term use. The goal is to avoid developing a situation where the drug ceases to work in the patient over time due to anti-drug antibodies or other reasons.
Phase 2 Combination Therapy Trials
As of now, several combination therapies are being investigated in ongoing trials. This is an exciting development in the field, and we anticipate more of these combined approaches to emerge in the near future.

Categorizing NASH Therapeutics by Mechanism of Action (MOA)
In the context of combination therapies for NASH, we can consider different categories, or “buckets”, of drugs based on their mechanism of action. These include agents that:
- Address overnutrition or endocrinopathies
- Focus on ER stress, lipotoxicity, or mitochondrial dysfunction
- Target inflammation, cholesterol toxicity, or have multifunctional roles
- Target fibrosis
Ideally, a combination therapy would include drugs from at least two of these categories. For instance, those in green hearts might be good potential combinations could involve Semaglutide, which addresses overnutrition; Lanifibranor and Resmetirom, which have multifunctional roles; and Obeticholic acid, which targets fibrosis.
Obeticholic acid may be particularly interesting for combination therapies as it has demonstrated fibrosis improvement properties. For example, pairing it with Resmetirom, a TRβ agonist that has shown positive effects on steatosis improvement and NASH resolution, could potentially synergize NASH resolution and fibrosis improvement. This combination could also provide additional benefits for pathogenic lipid improvement and might allow for a lower dose of Obeticholic acid, potentially reducing its adverse effects.
Future Therapeutic Considerations for Precision Medicine
Looking forward to a future of precision medicine, there are several potential pathways we could explore.
CAR-T therapy, traditionally associated with cancer treatment, could potentially be applied in advanced cirrhotic or fibrotic cases. One animal model study published in Nature in 2020 demonstrated promising results using CAR-T therapy to target senescent stellate cells, which produce fibrosis. Over time, this approach led to the resolution of scar tissue and improved liver chemistry tests.
Therapeutic oligonucleotides also offer an exciting avenue for targeted therapy. The DGAT2 antisense oligonucleotide, currently in Phase 2b trials, and the SERPINH1 (Heat shock protein 47) that targets fibrosis, are examples of this approach.
Further, the role of the microbiome in NASH is increasingly understood, opening up potential targets for therapy. Additionally, specific genetic polymorphisms like the HSD17B13 loss of sense mutation and PNPLA3 can be evaluated for targeted therapy.
In conclusion, the future of NASH therapeutics is moving towards targeted, personalized, and combination therapies that hold promise for more effective disease management and patient outcomes.




